
Fentons Reagent General Chemistry Using H2O2
Fentons Reagent General Chemistry Using H2O2
Fe 2+ + H2O2 → Fe 3+ + OH – + . OH
Fe 3+ + H2O2 → Fe 2+ + .OOH + OH
The procedure requires:
- adjusting the wastewater to pH 3-5;B
- adding the iron catalyst (as a solution of FeSO4); and
- adding slowly the H2O2. If the pH is too high, the iron precipitates as Fe(OH)3 and catalytically decomposes the H2O2 to oxygen — potentially creating a hazardous situation.
Reaction rates with Fenton’s Reagent are generally limited by the rate of . OH generation (i.e., concentration of iron catalyst) and less so by the specific wastewater being treated. Typical Fe:H2O2 ratios are 1:5-10 wt/wt, though iron levels < 25-50 mg/L can require excessive reaction times (10-24 hours). This is particularly true where the oxidation products (organic acids) sequester the iron and remove it from the catalytic cycle. Fenton’s Reagent is most effective as a pretreatment tool, where COD’s are > 500 mg/L. This is due to the loss in selectivity as pollutant levels decrease:
. OH + H2O2 → . O2H + H2O
. OH + Fe 3+ → Fe 2+ + OH –
In addition to free radical scavengers, the process is inhibited by (iron) chelants such as phosphates, EDTA, formaldehyde, and citric/oxalic acids. Because of the sensitivity of Fenton’s Reagent to different wastewaters, it is recommended that the reaction always be characterized through laboratory treatability tests before proceeding to plant scale.
Hydroxyl radical reactivity
The hydroxyl radical is one of the most reactive chemical species known, second only to elemental fluorine in its reactivity (see below).
| Reactive Species | Relative Oxidation Power (Cl2=1.0) |
|---|---|
| Flourine | 2.23 |
| Hydroxyl radical | 2.06 |
| Atomic oxygen (singlet) | 1.78 |
| Hydrogen peroxide | 1.31 |
| Perhydroxyl radical | 1.25 |
| Permanganate | 1.24 |
| Hypobromous acid | 1.17 |
| Chlorine dioxide | 1.15 |
| Hypochlorous acid | 1.1 |
| Hypiodous acid | 1.07 |
| Chlorine | 1 |
| Bromine | 0.8 |
| Iodine | 0.54 |
The chemical reactions of the hydroxyl radical in water are of four types:
Addition: . OH + C6H6 → (OH)C6H6 .
where the hydroxyl radical adds to an unsaturated compound, aliphatic or aromatic, to form a free radical product (cyclohexadienyl radical shown above).
Hydrogen Abstraction: . OH + CH3OH → . CH2OH + H2O
where an organic free radical and water are formed.
Electron Transfer: . OH + [Fe(CN)6] 4– → [Fe(CN)6] 3 + OH –
where ions of a higher valence state are formed, or an atom or free radical if a mononegative ion is oxidized.
Radical Interaction: . OH + . OH → H2O2
where the hydroxyl radical reacts with another hydroxyl radical, or with an unlike radical, to combine or to disproportionate to form a stable product.
In applying Fenton’s Reagent for industrial waste treatment, the conditions of the reaction are adjusted so that first two mechanisms (hydrogen abstraction and oxygen addition) predominate. Typical rates of reaction between the hydroxyl radical and organic materials are 109 – 1010 k (M-1 s-1).
Effect of Iron Concentration
In the absence of iron, there is no evidence of hydroxyl radical formation when, for example, H2O2 is added to a phenolic wastewater (i.e., no reduction in the level of phenol occurs). As the concentration of iron is increased, phenol removal accelerates until a point is reached where further addition of iron becomes inefficient. This feature (an optimal dose range for iron catalyst) is characteristic of Fenton’s Reagent, although the definition of the range varies between wastewaters. Three factors typically influence its definition:
- A minimal threshold concentration of 3-15 mg/L Fe which allows the reaction to proceed within a reasonable period of time regardless of the concentration of organic material;
- A constant ratio of Fe:substrate above the minimal threshold, typically 1 part Fe per 10-50 parts substrate, which produces the desired end products. Note that the ratio of Fe:substrate may affect the distribution of reaction products; and
- A supplemental aliquot of Fe which saturates the chelating properties in the wastewater, thereby availing unsequestered iron to catalyze the formation of hydroxyl radicals.
Iron dose may also be expressed as a ratio to H2O2 dose. Typical ranges are 1 part Fe per 5-25 parts H2O2 (wt/wt).
Effect of Iron Type (Ferrous or Ferric)
For most applications, it does not matter whether Fe2+ or Fe3+ salts are used to catalyze the reaction — the catalytic cycle begins quickly if H2O2 and organic material are in abundance. However, if low doses of Fenton’s Reagent are being used (e.g., < 10-25 mg/L H2O2), some research suggests ferrous iron may be preferred. Neither does it matter whether a chloride or sulfate salt of the iron is used, although with the former, chlorine may be generated at high rates of application.
It is also possible to recycle the iron following the reaction. This can be done by raising the pH, separating the iron floc, and re-acidifying the iron sludge. There have been some recent developments in supported catalysts that facilitate iron recovery and reuse.
Effect of H2O2 Concentration
Because of the indiscriminate nature by which hydroxyl radicals oxidize organic materials, it is important to profile the reaction in the laboratory for each waste to be treated. For example, in a typical application the following series of reactions will occur:
| Substrate | → | Oxidized Intermediate “A” | → | Oxidized Intermediate “B” | → | Oxidized Intermediate “C” | → | Oxidized Intermediate “D” | → | Oxidized Intermediate “E” | → | CO2 |
Each transformation in this series has its own reaction rate and, as the case of phenolics illustrates, there may occur build-up of an undesirable intermediate (quinones), which requires sufficient H2O2 to be added to push the reaction beyond that point. This is frequently seen when pretreating a complex organic wastewater for toxicity reduction. As the H2O2 dose is increased, a steady reduction in COD may occur with little or no change in toxicity until a threshold is attained, whereupon further addition of H2O2 results in a rapid decrease in wastewater toxicity.
Effect of Temperature
The rate of reaction with Fenton’s Reagent increases with increasing temperature, with the effect more pronounced at temperatures < 20 deg-C. However, as temperatures increase above 40-50 deg-C, the efficiency of H2O2 utilization declines. This is due to the accelerated decomposition of H2O2 into oxygen and water. As a practical matter, most commercial applications of Fenton’s Reagent occur at temperatures between 20-40 deg-C.
Applications of Fenton’s Reagent for pretreating high strength wastes may require controlled or sequential addition of H2O2 to moderate the rise in temperature which occurs as the reaction proceeds. This should be expected when H2O2 doses exceed 10-20 g/L. Moderating the temperature is important not only for economic reasons, but for safety reasons as well.
Effect of pH
The effect of pH on reaction efficiency is illustrated below:

The optimal pH occurs between pH 3 and pH 6. The drop in efficiency on the basic side is attributed to the transition of iron from a hydrated ferrous ion to a colloidal ferric species. In the latter form, iron catalytically decomposes the H2O2 into oxygen and water, without forming hydroxyl radicals. There have been some recent developments using nonradical scavenging sequestering agents (e.g., NTA and gallic acid) to extend the useful pH range to pH 8-9, but no commercial applications are known. The drop in efficiency on the acid side is less dramatic given the logarithmic function of pH, and is generally a concern only with high application rates.
A second aspect of pH deals with its shift as the reaction progresses. Provided an initial wastewater pH of 6.0, the following profile is typical of Fenton reactions.
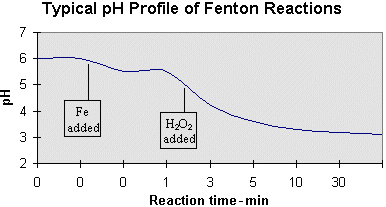
The first inflection is caused by the addition of FeSO4 catalyst which typically contains residual H2SO4. A second, more pronounced drop in pH occurs as the H2O2 is added, and continues gradually at a rate which is largely dependent on catalyst concentration. This drop in pH is attributed to the fragmenting of organic material into organic acids. This pH change is often monitored to ensure that the reaction is progressing as planned — the absence of such a pH decrease may mean that the reaction is inhibited and that a potentially hazardous build-up of H2O2 is occurring within the reaction mixture.
In highly concentrated waste streams (>10 g/L COD), it may be necessary to perform the oxidation in steps, readjusting the pH upwards to pH 4-5 after each step so as to prevent low pH from inhibiting the reaction.
Effect of Reaction Time
The time needed to complete a Fenton reaction will depend on the many variables discussed above, most notably catalyst dose and wastewater strength. For simple phenol oxidation (less than ca. 250 mg/L), typical reaction times are 30 – 60 minutes. For more complex or more concentrated wastes, the reaction may take several hours. In such cases, performing the reaction in steps (adding both iron and H2O2) may be more effective (and safer) than increasing the initial charges.
Determining the completion of the reaction may prove troublesome. The presence of residual H2O2 will interfere with many wastewater analyses. Residual H2O2 may be removed by raising the pH to e.g., pH 7-10, or by neutralizing with bisulfite solution. Often, observing color changes can used to assess the reaction progression. Wastewaters will typically darken upon H2O2 addition and clear up as the reaction reaches completion.
Effect of Post Treatment
As a result of degrading complex organic materials into organic acid fragments, the pre-oxidized effluent is generally more amenable to conventional treatment, e.g., flocculation and biotreatment. The presence of iron in the reaction mixture makes it particularly suited to subsequent lime flocculation. In many cases, it may be possible to remove up to 80% of the wastewater COD through a combination of Fenton’s Reagent and lime flocculation. Significantly, this may be achieved with an H2O2 dose of 50-75% of the stoichiometry.
Process Equipment
Historically, Fenton’s Reagent has been applied in batch mode. Today, however, it is being used in both continuous and sequential batch processes. A typical batch operation would consist of chemical storage and dosing modules (for H2O2, FeSO4, acid, and lime/NaOH); a primary reactor and (optional) holding tank; a solids dewatering device (optional); and miscellaneous temperature and pH controls. The materials of construction for the reactor and holding tank are typically Types 304 or 316 stainless steel, while those for the chemical storage systems may also be HDPE. Packaged Fenton’s systems are available from some equipment providers, or US Peroxide can provide you with engineering guidance on custom designs and retrofits.
References
Bishop, D.F. et.al. “Hydrogen Peroxide Catalytic Oxidation of Refractory Organics in Municipal Waste Waters”, in Ind. Eng. Chem., Process Design & Development, vol.7, pp. 1110-117 (1968).
“Fenton’s Reagent Revisited”, in Accts of Chem. Research, vol. 8, pp. 125-131 (1975).
USP Technologies
5640 Cox Rd.
Glen Allen, VA 23060
Toll-free (800) 851-8527
Phone (804) 404-7696
USP - Canada
3020 Gore Road
London, Ontario N5V 4T7
Toll-free (800) 851-8527
Phone (804) 404-7696
Contact Us
Our sales engineers are ready to help you find the right solution. Fill out the form to connect with your local USP Technologies representative.
"*" indicates required fields