
H2O2 Self-Accelerated Decomposition
H2O2 Self-Accelerated Decomposition
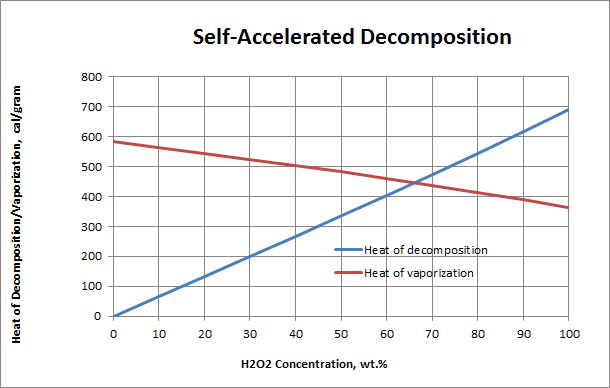
Notes:
- H2O2 decomposition is highly exothermic (23.44 kcal/mole). Even 10% H2O2 can boil if it becomes grossly contaminated.
- The effect of temperature is such that an increase of 10 °C increases the rate of decomposition by a factor of 2.3 (i.e., a first order rate equation). Therefore, decomposition can accelerate if the solution becomes grossly contaminated.
- As the concentration of H2O2 in solution increases, there is less water to absorb the heat of decomposition. A crossover occurs at 63-64% H2O2 where rapid, accelerated decomposition becomes self-sustaining and the concentration of H2O2 in the decomposing solution can actually increase.
USP Technologies
5640 Cox Rd.
Glen Allen, VA 23060
Toll-free (800) 851-8527
Phone (804) 404-7696
USP - Canada
3020 Gore Road
London, Ontario N5V 4T7
Toll-free (800) 851-8527
Phone (804) 404-7696
Contact Us
Our sales engineers are ready to help you find the right solution. Fill out the form to connect with your local USP Technologies representative.
"*" indicates required fields