
Struvite Reduction via H2O2 and Ferric Chloride
Background
In 2017, JEA was presented with two methods to control struvite formation at several points in their biosolids handling system at the wastewater treatment plant. One method utilized ferric iron (as FeCl3) to complex with phosphorous to interrupt the struvite potential, and the other utilized ferrous iron (as FeCl2) in combination with hydrogen peroxide to remove sulfides more efficiently, then convert the iron to ferric using peroxide for removal of phosphorous. The ferric chloride approach was chosen, and JEA has utilized an iron-only program to control struvite for 4 years at the wastewater plant. While effective, the program required an excessive amount of the iron solution be fed to an area susceptible to corrosion, forcing the plant to deal with replacing assets damaged by the localized acidic conditions. In addition, the struvite control was sporadic, offering a small margin of error when the treatment was not fed continuously or underfed. If the program lapsed or was poorly controlled, the piping from the pumps to the diversion valve would decrease in cross sectional area, taking the 8-inch pipe to an effective diameter of 2-inches over the course of 10-14 days. As an aside, it is necessary to send the centrate through a dedicated side-stream biological treatment before blending with the main water from the primary clarifier to make this stream more acceptable to the secondary treatment plant. This side-stream treatment must be by-passed when the drain line is plugged, resulting in higher loading to the biological plant, labor intensive efforts to clear the rock-hard scale, and a potential temporary shutdown of the centrifuge system while the work is done. As a solution to this problem, USP Technologies recommended utilizing peroxide at key points to form ferric iron and address the problem both upstream of the digester, and at the centrifuge to remove phosphate from the liquid streams at two key points where the formation of struvite was an issue.
Results
The use of peroxide for converting ferrous iron to ferric iron to benefit the removal of phosphorous at key points of the system was accomplished by installing a peroxide storage tank and dual pump dosing skid at the plant. One pump fed peroxide to the raw sludge feeding the Gravity Belt Thickeners to oxidize sulfides and remove them from the liquid stream, creating fresh iron for re-use. The other pump feeds peroxide to the digestate from digested sludge tank upstream of the centrifuge to reform the iron phosphate complex by forming ferric iron, removing phosphorous with the solids and disrupting the struvite potential in the centrate.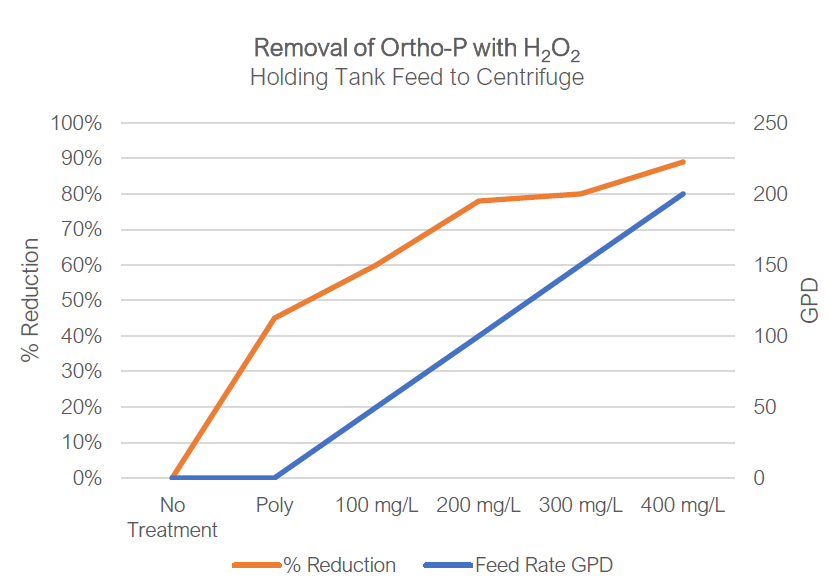
The results have repeatedly shown that a modest amount of hydrogen peroxide can be utilized to effectively remove >90% of the phosphate in the system, effectively preventing formation of struvite in the centrate line of the wastewater plant. Target removal is >82% which has been shown sufficient to maintain a clean line.
The use of peroxide to regenerate iron to the ferric form can be successful in dramatically reducing the potential for struvite formation with little capital investment. The benefits of the program extend to other areas beyond runability, impacting safety, digester gas quality, and asset preservation.
To read the entire case study, click here.
Featured Product

PRI-TECH®– Peroxide Regenerated Iron
PRI-TECH® is an innovative combination treatment that integrates iron salts with hydrogen peroxide (H2O2) in a unique and synergistic fashion, resulting in cost savings and treatment enhancement compared to traditional iron salt use.
USP Technologies
5640 Cox Rd.
Glen Allen, VA 23060
Toll-free (800) 851-8527
Phone (804) 404-7696
USP - Canada
3020 Gore Road
London, Ontario N5V 4T7
Toll-free (800) 851-8527
Phone (804) 404-7696
Contact Us
Our sales engineers are ready to help you find the right solution. Fill out the form to connect with your local USP Technologies representative.
"*" indicates required fields